Autism
The Metabolic Basis of Autism Spectrum Disorder (ASD)
ASD is a Multisystem Disorder: The Medical Complexity Suggests a Systemic Etiology
Although ASD is traditionally thought to only involve the brain, over the last few decades it has been appreciated that 95% or more of individuals with ASD have at least one comorbid medical diagnosis [1]. One study identified three patterns of comorbid conditions affecting individuals with ASD [2]. About half were found to have a low number of comorbid conditions, with these comorbid conditions occurring with a similar prevalence to the general population. About a quarter of ASD individuals demonstrated a medium number of comorbid conditions, particularly developmental delays and auditory conditions. Lastly, a quarter manifested many comorbid conditions, with immune, GI, and psychiatric conditions being most prevalent. This suggests that there are at least a quarter of individuals with ASD who have a multi-system presentation.
A Mitochondrial Etiology Can Explain the Medical Complexity of ASD
Mitochondrial disorders are characterized by multisystem dysfunction [3], particularly affecting the brain [4] and gastrointestinal system [5], two systems that are commonly affected in ASD [6]. In addition, it is becoming clearer that those with mitochondrial disorders also have functional immune disorders, making them susceptible to infections and sepsis [7,8]. Many children with ASD demonstrate immune dysfunction, including early recurrent treatment resistant infections as well as autoimmunity [9]. Of note, major diagnostic criteria for diagnosing mitochondrial disease, the modified Walker [10] and the Morava [11] criteria, heavily weigh multisystemic presentation in the diagnosis of mitochondrial disease.
Systems Biology of Autism Spectrum Disorder (ASD): The “Terrible Trio”
One of the reasons that the underlying biology of ASD may be difficult to understand is the fact
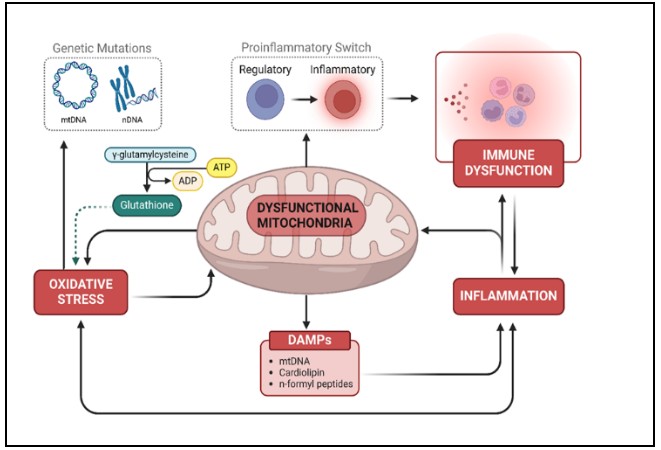
that it involves the interaction between multiple biological systems [12,13] (see right figure). One systematic review demonstrated that multiple brain studies have linked the major systemic abnormalities involved in ASD: mitochondrial and immune dysfunctions and oxidative stress, also known as the “terrible trio” [13]. These processes are not isolated phenomena; rather, they form self-perpetuating feedback loops that drive the metabolic and neurological disruptions observed in ASD. Thus, central to our understanding of ASD is the dynamic interplay of three interconnected factors.
Mitochondria May Mediate Prenatal Environmental Influences in ASD
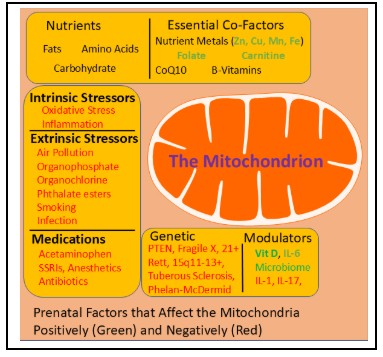
Many prenatal factors associated with an increased risk of ASD are associated with mitochondrial dysfunction (see left figure). These include environmental toxicants, including air pollution, nutritional agents, both intrinsic and extrinsic stressors, common medications given during pregnancy, modulators of mitochondrial function, genetic conditions which might affect the fetus and alternations in the microbiome [14]. In fact, prenatal exposure in rodent models of immune activation (MIA) results in long-term alterations in mitochondrial function [15,16]. Several studies have now shown that alterations in mitochondrial and fatty acid metabolism are neonatal markers of increased ASD risk [17].
Early Environmental Exposures Predispose to Neurodevelopmental Regression (NDR)
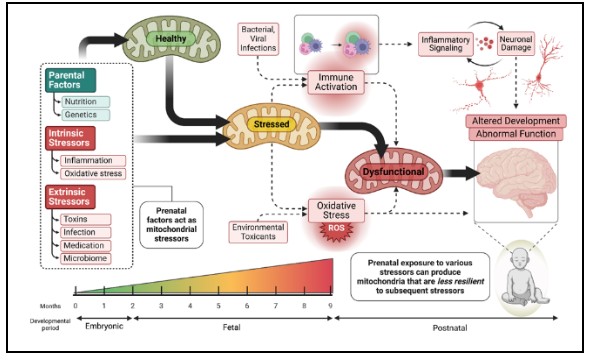
Recent studies have demonstrated that NDR is associated with a specific type of mitochondrial dysfunction [18]. In classic mitochondrial disease, mitochondrial respiration is depressed, whereas in ASD children (and their parents), mitochondrial respiration is elevated to twice that of controls. This is consistent with our lymphoblastoid cell model of mitochondrial dysfunction where we find this pattern is associated with mitochondria that are more vulnerable to physiological stress [17]. We have found that this pattern of mitochondrial abnormalities are linked to prenatal exposure to air pollution [19] and deficiencies in prenatal nutrition metals zinc and manganese [20]. Thus, we believe the prenatal environment alters the mitochondria to become vulnerable to postnatal physiological stresses, resulting clinically in what we see as NDR (see figure left).
Cerebral Folate Insufficiency: A Convergence of the Terrible Trio
Over the past decade we have shown that leucovorin (folinic acid) has a significant therapeutic effect on children with ASD who have problems transporting folate into their nervous system using the primary transport mechanism, the folate receptor alpha [21]. This can be the result of several non-exclusive mechanisms. Our meta-analysis found that 71% of children with ASD have folate receptor alpha autoantibodies which bind onto the transporter, reducing its function [22]. We also found that cerebral folate deficiency was caused by the folate receptor alpha autoantibodies 83% of the time, mitochondrial dysfunction 43% of the time and genetic mutations 14% of the time [22]. Thus, two of the main mechanisms of the terrible trio converse on a single mechanism that can result in ASD symptoms.
Addressing Mitochondrial Dysfunction to Improve Multisystem Function
Mitochondria are at the heart of metabolic systems, affecting and being affected by metabolic disruptions. Several controlled trials have demonstrated that common mitochondrial support supplements such as carnitine and coenzyme Q10 (CoQ10) can improve symptoms in ASD [17]. A recent double-blind placebo controlled crossover trial of a supplement cocktail designed to target dysfunctional mitochondrial and metabolic systems in ASD was found to not only improve ASD symptoms but to also improve mitochondrial resilience to physiological stress [23]. The Ketogenic Diet, a diet traditionally used for drug-resistant epilepsy, has been shown to improve both ASD symptoms and mitochondrial function [24]. Mitochondrial dysfunction can drive inflammation in the brain and body, thus, treatment, especially with the Ketogenic Diet can improve both mitochondrial function [25] and reduce neuroinflammation [26].
Glutathione Deficiency, which is Central to Metabolic Resistance, can be Improved with Safe Treatments.
Abnormalities in the transsulfuration and transmethylation pathways are so common in ASD that they could be diagnostic [27,28]. Abnormalities in these pathways lead to glutathione deficiency syndrome [29], leading to vulnerability to physiological stress, immune activation and environmental toxicants. Glutathione can be normalized in children with ASD using subcutaneous injections of methylcobalamin and leucovorin (folinic acid), leading to improvements in ASD symptoms as well as methylation and oxidative stress [30].
Transgenerational Inherited Disorders Disrupt Prenatal and Postnatal Development
Of significant interest is that the same physiological abnormalities found in the children with ASD, including mitochondrial dysfunction [18], transsulfuration / transmethylation abnormalities [31] and immune activation [32], are also be found in the parents and siblings, suggesting complex systematic biological abnormalities that are inherited in a non-Mendelian manner. This raises the possibility that such abnormalities may be identified prenatally or preconception and treated to prevent ASD from developing, thus reversing the current increasing ASD trend. Leucovorin (folinic acid) has been shown to reverse the behavioral deficits of prenatal exposure to maternal folate receptor alpha antibodies. [33]. Other simple and safe treatments could provide the same protection prenatally.
Key Elements of the Systems Biology Model in ASD:
- Neuroinflammation and Immune Dysfunction
- Mitochondrial Dysfunction
- Oxidative Stress and Glutathione Deficiency
Opportunities for Intervention and Prevention:
- Early Detection
- Systems-Based Treatments
Conclusion
ASD must be reframed as a systems biology disorder with modifiable metabolic and immune drivers. Recognition of the “terrible trio” of mitochondrial dysfunction, glutathione deficiency and neuroinflammation as root causes opens new avenues for prevention, treatment, and recovery. With early identification and comprehensive intervention, we can disrupt the feedback loops that sustain this disorder and offer hope for improved outcomes and quality of life for affected individuals and their families. Additionally, investigating these systemic abnormalities in a transgenerational fashion can lead to prevention of childhood chronic disease.
Dr. Richard Frye, a pediatric neurologist and autism expert, fully employs metabolic therapy to transform the lives of children with autism, leveraging cutting-edge research into mitochondrial function and nutritional interventions as Director of Autism Research at Rossignol Medical Center.
Article Reference: Autism – References